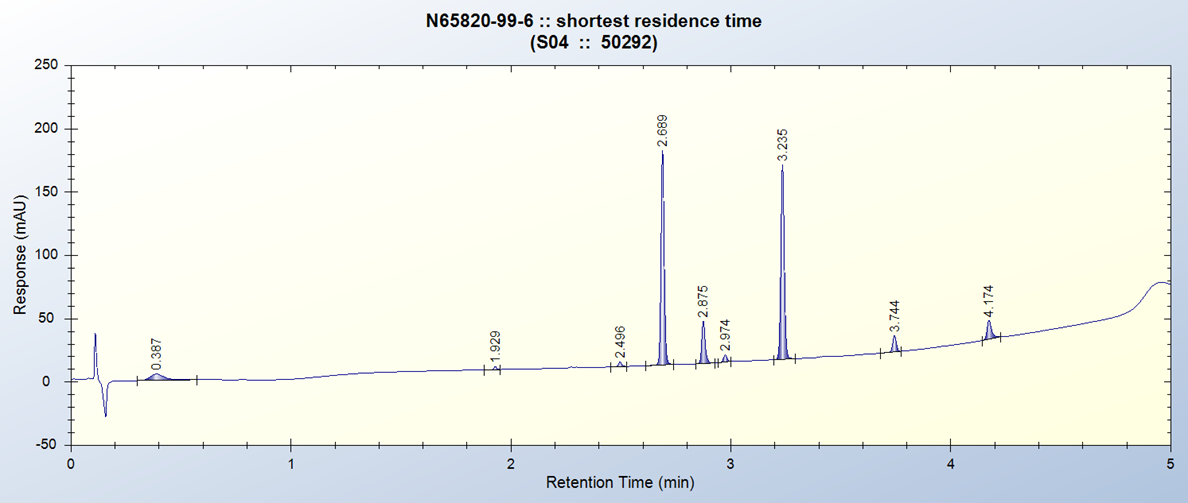
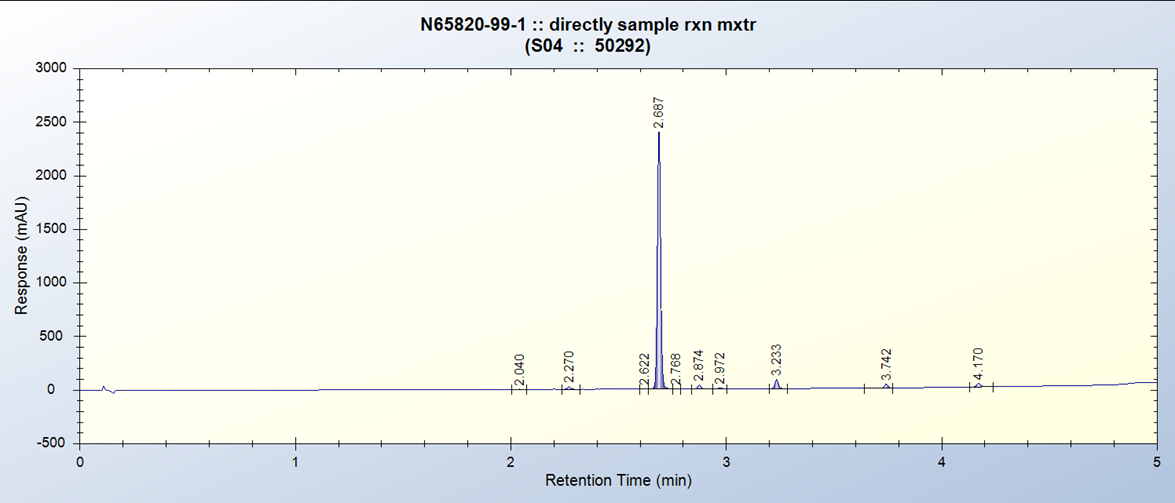
Project Example:- Ester Reduction
Reactions typically complete after 2 h with above conditions in batch.
Alcohols tend to precipitate out of solution as reaction progresses.
54 minute residence time DART-DM7 Flow Reactor.
DART-DM reactors can be configured to meet the needs of many different synthetic and biological processes.
DART-DM reactors can process reactions involving gases, liquids and suspended solids, (slurries). Photochemistry versions of DART-DM are now also available opening up new areas of processing and manufacturing where catalysts are used in photochemistry.
With the kind permission of our customers; below are just a few examples of the types of reactions that can be undertaken in DART.
5 independent HTF zones
Hastelloy or Stainless Steel
Choice of drives, automation and instrumentation
5 Independent HTF zones
Hastelloy or Stainless Steel
Chioce of wavelengths.
Single tube design
Hastelloy or Stainless Steel
2 Working volumes of 60 and 155ml
2 Heat transfer zones
Handles highly viscous materials and high solids concentrations.
Single tube design
Hastelloy or Stainless Steel
Taylor Vortex Mixing
Gas membrane for safe handling of O2
2 Electric Heat transfer zones
Hastelloy or Stainless Steel
2 independent Heat transfer zones
Choice of Drives, automation and instrumentation
Photochem module option
2 Heat Transfer Zones
Hastelloy or Stainless Steel
14ml Working volume
Handles viscous material and high solids concentrations
Electrically heated
PEEK and Borosilicate Glass
Taylor Vortex Mixing
7ml Working volume
2 Heat Transfer Zones
Hastelloy or Stainless Steel
14ml Working Volume
Chioce of drives automation and instrumentation
Photochem module option
25ml Working Volume
PTFE/PFA/Borosilicate Glass construction
Photochemistry with suspended solids.
500ml nominal working volume
Hastelloy or Stainless Steel
Project Example:- Ester Reduction


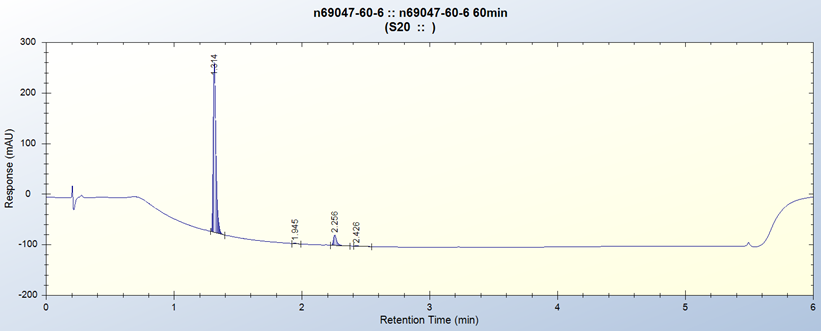
Project Example:- NO2 Reduction
30min Residence Time in DART-DM7x7 Flow Reactor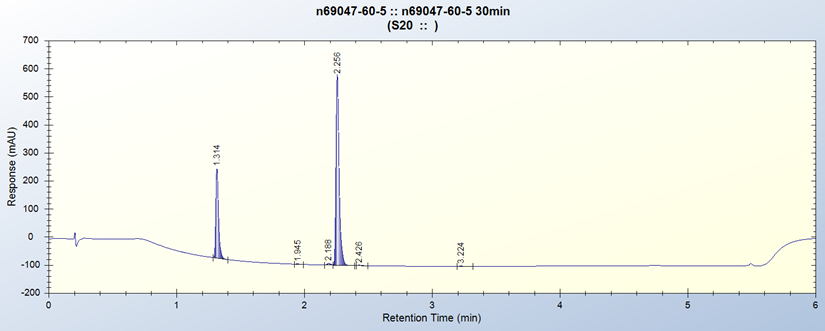
It is widely known that that a high CO2 mass transfer rate is vital to the
design of better bioreactors for CO2
mitigation. By comparison with traditional sparging methodologies, the use of a DART reactor greatly increases the residence time of the gas in the algae culture.
Ruthenium-Catalyzed Ester Reductions Applied to Pharmaceutical Intermediates
https://www.nature.com/articles/s41467-019-13988-4
Carbon Capture
https://www1.chester.ac.uk/autichem-ltd
Teaching and learning
https://link.springer.com/article/10.1007/s41981-020-00118-1